We spent our second Sunday in a row at the Ray Mine. You can read last Sunday'sfield trip report by clicking on the following link: October 17, 2004 Ray Mine Report
The weather forecast that called for amostly sunny sky and a high temperature of 72-degrees was too hard to resist, as was the call of the rocks: Mike, Chrissy, Mike, Chrissy, Mike, Chrissy . . . . come . . . come . . . come . . .
We found more of the usual Ray Mine rocks and minerals, including beryl, amazonite, aquamarine, mica, quartz, schorl, and apatite. The amazonite was particularly good as I had stumbled upon an area in the spoil piles that contained more in one place than I had ever seen, including many euhedral crystals in matrix. We also found several small smoky quartz crystals.






But, that was not the best part. While busting rocks, I found some excellentpinkish-purple massive fluorite. On other occasions at the mine, I had recovered fluorite specimens as small blebs and thin veins; but, on this day, the massive fluorite areas were much larger and more colorful.





But, that wasn't the best part either. As I continued to cob rocks, I came upon a splash of color and crystalline structure that I had not seen before at the Ray. Somehow I had, by chance, managed to discover a variety of fluorite called chlorophane! To my knowledge, this is the first identification of chlorophane at the Ray Mine!!!
Chlorophane is a relatively rare variety of fluorite and is remarkable because it displays fluorescence, phosphorescence, and thermoluminescence. The chlorophane that I found at the Ray fluoresces greenish white under short wave ultraviolet light. When illuminated with a bright visible light source, and then viewed in darkness, chlorophane displays a lingering green to blue glow. This phosphorescent glow can last for many minutes. I heated up some tiny chips of chlorophane using a skillet on the stove and they glowed a bright bluish/greenish white.

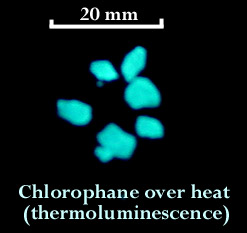
I have read that chlorophane will thermoluminescence only one time and after the glow fades it will not be seen again in the same specimen. I tested this theory and found that my chlorophane must not have gotten the memo because it lit up a second time when heated in the pan. I also read that chlorophane is found in very limited quantities at Amelia Court House, Virginia; Franklin, New Jersey and the Bluebird Mine, Arizona; Gilgit, Pakistan; Mont Saint-Hilaire, Quebec, Canada and at Nerchinsk in the Ural Mountains, Russia. (http://mineral.galleries.com)


I believe I was in a unique position to first identify chlorophane at the Ray because I'm a professional geologist with lots of Ray Mine digging experience and I had seen the mineral in person a couple times at the McHone mine in Spruce Pine, NC where it is abundant. If not for these factors, it would have been easy to toss the Ray chlorophane aside because, at a quick glance, it resembles highly fractured smoky quartz, especially when mostly covered in dirt. I showed the chlorophane I had found to Carl Merschat with the NC Geological Survey and we surmised it likely exists in other Spruce Pine District pegmatites, but has yet to be recognized.
According to Dr. Bill Miller, Professor of Geology at the University of North Carolina at Asheville, "Your discovery of chlorophane is indeed interesting. You may know that chlorophane is a synonym for fluorite, which has been reported by Kenth & Kerr and found by others more recently. I do not recall Kenth & Kerr's description of the fluorite, but my student, Curtis Allen, described it as small, massive, blue inclusions in albite. He didn't say his fluorite was fluorescent, but perhaps you have found another variety." As it turns out, my identification of chlorophane was a first for the Ray Mine. I am thrilled to have found chlorophane at the Ray and you can bet that I'll be keeping my eyes open for more there and elsewhere.